| The
quest for a viral victory
By Lindsay Chung
OTTAWA —
All around the world, young
boys are slowly wasting away from Duchenne Muscular
Dystrophy. There is still no cure for this debilitating
neuromuscular disease, and scientists have been working
feverishly for years to come up with a way to combat
the effects that leave most patients in a wheelchair
before the age of 10.
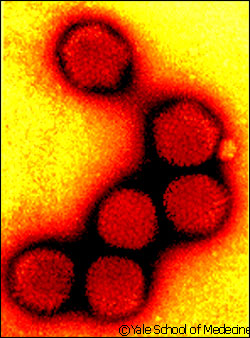 |
| Adenoviruses
can be used to inject genes into cells to produceprotein. |
Dr. Robin Parks has muscled his way into
the arena with his innovative research into viral vectors.
Parks, a molecular scientist at
the Ottawa Health Research Institute, studies the use
of adenoviruses as a way to deliver therapeutic genes
into animal models of genetic or acquired diseases.
An adenovirus is a non-enveloped,
spherical virus containing double-stranded DNA. Adenoviruses,
which cause respiratory diseases such as the common
cold, are attractive vectors, or vehicles, for delivering
foreign genes into animal cells because they have an
increased cloning capacity.
Since he started his own lab at
the Research Institute in 1999, he has worked on many
aspects of gene therapy, including a way to reverse
the effects of Duchenne Muscular Dystrophy (DMD).
Parks is also an assistant professor
in the University of Ottawa’s department of medicine
and biochemistry. In 1996, while working on his post-doctoral
fellowship with Dr. Frank L. Graham in the department
of biology and pathology at McMaster University, Parks
started using adenovirus vectors in gene therapy and
continued it once he joined the Research Institute.
The disorder
DMD, a degenerative muscle disorder
that affects about one in 3,500 men, is caused by genetic
malfunctions. People born with DMD have a mutation in
their dystrophin gene, which results in a lack of dystrophin
protein. This protein is important for the normal function
of cells that are required during muscle contraction
and stretching. Since dystrophin is missing, the muscle
cells are weaker and tear faster than the body can repair
them. As their muscles waste away, patients end up in
wheelchairs and eventually succumb to respiratory or
cardiac failure because the muscles of the diaphragm
and heart stop working.
According to George Henderson, national
manager of firefighter relations and communications
for Muscular Dystrophy Canada, the diagnosis generally
occurs at the ages of two or three, he says. Life expectancy
for those suffering from the disorder has changed dramatically
over the years.
“In 1954, when we were first
formed, expectations were until the early teens,”
he says. “The great progress in the last 50 years
has improved their mobility and quality of life and
has extended their life to allow these individuals to
live much longer lives.”
“We now have a number of clients
in their 40s,” he adds. “They are completely
paralyzed and require breathing assistance.” Life
expectancy for DMD patients is now well into the 20s.
However, there is still no cure for the disease.
The research
This is where Dr. Parks comes in.
Parks had previously been working with viruses before
he became interested in muscle disease. He says there
are a lot of people at the Research Institute and the
University of Ottawa who are very knowledgeable about
muscle diseases, so he and his research partners latched
onto this group.
Parks and his team are working on
adenoviruses that have been stripped of all their viral
genes, known as helper-dependent adenoviruses.
Parks' assistant Robert Lanthier
says to get rid of viral RNA, the virus is run on a
gradient, which is a grainy solution that is put into
a tube with the virus. The tube spins really fast in
a centrifuge, and the virus is separated into various
components based on size.
When the virus is injected into
the animal, it binds onto the outside of the cell by
attaching to the cell’s receptor proteins. It
then moves inside to take over the cell and replicate
itself.
The researchers give the virus
a week or so to run its course, and then they kill the
mouse and harvest some of its cells. They compare the
cells from a mouse infected with the disease and a healthy
one, in order to see if the injected viral genes have
had any benefit and whether they have succeeded in producing
the missing protein.
A virus is full of genes, which
code for proteins, so if a person has a bad copy of
the gene they are not able to make a certain protein,
Lanthier explains. When the virus takes over the cell,
it exploits the cell’s machinery for making the
protein, and the virus forces the cell to make protein
for it. Once the virus has made its way into the cell,
it breaks apart and exposes its DNA, and there are proteins
in the cell that make copies using the virus’
RNA.
As Lanthier explains, DNA is like
the whole recipe book, made up of smaller fragments.
The genes are the recipes, and they are each a code
for something specific. The cell reads the recipe, and
the final product is a protein. Researchers hope that
this protein will be as successful as a well-baked chocolate
cake.
“If a virus makes more copies
of itself, the immune system can tell something is going
on, so we remove all the viral genes, and that way the
system doesn’t detect it,” Parks explains.
“In a variety of disease models,
it seems to work fairly well,” he says. “It’s
not perfect, but much better than other virus vectors
that people are using.”
The helper-dependent adenoviruses
have potential in gene transportation because they have
a large capacity for cloning and they may allow for
the simultaneous delivery of multiple genes.
Parks has also removed the essential
genes, known as early region 1 (E1), from the adenovirus.
These E1 genes are required for normal virus replication;
therefore, the virus cannot cause disease in humans
because it can only grow in the lab.
“In animal models of DMD,
these viruses have shown to work quite well and can
reverse the DMD state,” Parks says.
The catch
However, there is a catch. A
person’s immune system is very good at detecting
when a cell has been infected with a virus, Parks explains.
While the adenovirus lacks the E1 genes, there are still
numerous viral genes present, and they produce a bit
of virus protein that is detected by the body’s
immune system.
The immune system will attack and
eliminate the cell that has been infected by the virus,
regardless of whether the virus is producing a therapeutic
gene or not. To counteract this, Parks has removed all
the viral genes from the virus so that there is nothing
for the immune system to detect. This is what makes
his research unique among the various scientists studying
gene therapy for DMD.
“Any cell infected with these
new and improved cells will no longer be detected as
being infected, and, hopefully, the dystrophin gene
will provide enough protein to correct the DMD state,”
he says, adding that these new viruses have proven to
be much more effective in animals than previous viruses.
Along the way, Parks has come up
against many obstacles.
“We’ve discovered a
few things about how the cell responds (to the virus),”
Parks says. “Not only the immune system responds,
but all the cells respond by signals.”
“We’re now trying to
figure out how we blunt some of these signals so the
virus hides easier.”
Parks says his research has uncovered
some interesting facts. He has noticed how viruses have
evolved to try to take over a cell and the cell, in
turn, has advanced to withstand the virus.
“It’s interesting how
the two have co-evolved to try and combat each other,”
he says.
The community
Parks stresses that he is only one
researcher in a whole community of scientists looking
at DMD. They share a common goal of finding new therapies
for the disorder. He says his lab keeps in contact with
Muscular Dystrophy Canada, as well as the Muscular Dystrophy
Association of the United States in order to keep up
to date and help one another.
For example, Dr. Parks and Dr. Jonathan
Bramson, who works out of the Centre for Gene Therapeutics
at McMaster University, recently received a grant from
Muscular Dystrophy Canada for their project “Building
a Better Vector.”
“We keep in touch with people
affected with the disease,” he says. “It
more or less keeps it real for us, so we know what we’re
trying to cure.”
Teren Clarke, national director
of programs and services at Muscular Dystrophy Canada,
is in charge of providing research grants to scientists.
She says projects such as Parks’s have been receiving
a lot of money lately because the hope for hereditary
neuromuscular disorders like DMD lies in gene therapy
or stem cell therapy.
In order to receive a grant, the
research proposal must be peer-reviewed and must be
judged as high quality science with relevance to people
with neuromuscular disorders, Clarke explains.
Parks has to submit a report each
year. Clarke says they have been very satisfied with
his work, but they know a cure is still a long way off.
“He’s continuing to
add to the body of knowledge,” she says. “But
we’re certainly not close to a cure or therapy.
It could be considered as a small step in the journey
to a cure.”
"The more (the scientists)
learn, the more they uncover that we don’t know.”
|