|
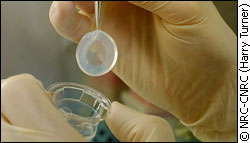
Now scientific research seeks to help the body rebuild itself. "The research is in the area of regenerative medicine and particularly we’re interested in tissue engineering," says Dr. May Griffith, a cell biologist with the Ottawa Eye Institute. Researchers are using polymers to create a structure that cells can easily grow in and regenerate tissue. "Polymer is just a very very big molecule, which is nothing new,” Dr. David Carlsson says, an Emeritus researcher with the National Research Council. “Nature has been making these things for millions and millions of years." Natural and synthetic Synthetic polymers include plastics, ranging from toothbrushes to cell phones. Cushions and Styrofoam are examples of polymer foams. Polymers also occur naturally in the body, in collagen and starch. Natural polymers are useful for tissue engineering because they’re biodegradable. Body parts The World Health Organization estimates that 10 million people are in need of cornea transplants to prevent blindness. The cornea is the transparent layer over the front of the eye. It directly covers the iris, the coloured part of the eye. The cornea becomes cloudy with age, disease or injury. "It’s responsible for about 80 per cent of the light transmission into the eye," Griffith says. "So that’s why if it’s cloudy the vision is obscured." Seeking sight Dr. Griffith and her team created a polymer cornea implant that helps patients generate a new cornea. It looks much like a contact lens. "So what we do is use either natural polymers, like collagen or a mixture of natural and synthetic polymers, mix them up, put them into a mold, then mold them into the shape of a cornea. And so these are just transplanted into animals," Griffith says. The goal is to make an implant that closely resembles a human cornea. "We aim for the same properties, so you know [the] same transparency, same water content, and then to that we add …bioactive peptides…that encourages nerve growth," Griffith says.
"But you know whatever you put into it will depend on what condition you’re trying to treat." The transplants have been tested in mice, rats and rabbits, Griffith says. The treatment for corneal disease is transplantation from human tissue donation. But Griffith hopes that some of her transplant models will be available to people in the next few years. "There are some that are almost ready to go into humans right now; we just probably need to get through all that paper work." A step further Joint replacement is one of the top three areas where treatment is most needed, according to the Canadian Institutes of Health Research. Knee problems are very common, Carlsson says, because cartilage in the knee gets worn out with age. "It will start to break down, finally you’ll get bone to bone contact and you get intense pain. So people cannot walk properly and they lose mobility," he says. The treatment for a knee replacement now is basically to replace this very complex joint with a mechanical hinge. "It’s still a prosthesis— an artificial device going into your body, nothing like the original tissue," Carlsson says.
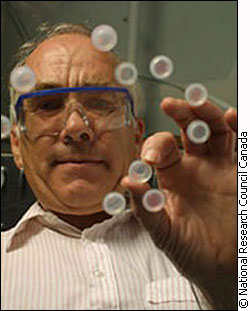
"It’s known already that the body can regenerate, meaning repair, rebuild, a lot of parts of your body if you could only find out how to help the body do that," Carlsson says. "But could you get the cells in your knee which can build this material we call cartilage…and restore it just the way it was?" The answer isn’t so simple. "Theoretically you can do it. Technically it’s a huge problem," says Dr. Carlsson. The trick is to create the right environment for the cells to grow in. The cells you’re working with will depend on the kind of tissue you wantto grow, and different cells thrive in different environments. Dr. Victoria Nawaby, of the National Research Council, uses gases to create certain structures with polymers. "We’re working on a corn based polymer, a polymer that is obtained from a natural resource rather than a petrochemical base," she says. A natural based polymer is more compatible with the human body and can be used to create a scaffold to grow cells on. Building a dream home An ideal scaffold for cell growth is like an apartment complex with doors connecting each separate unit. The cells would be placed in each apartment and begin to multiply and connect with their neighbouring cells to eventually form a tissue. But the original scaffold will eventually disappear if it’s made of biodegradable material. "Now [if] this apartment complex is biodegradable eventually these guys will use this as food and they will grow enough and become a healthy tissue and they take over on their own," Nawaby says.
"My area is to design this apartment complex," Nawaby says. This complex is called a polymeric cellular morphology or foam. "An example of a foam is a Styrofoam cup. But the cell size in that is very large, you break it and you can literally see the cavities in between," she says. The foams Nawaby creates cannot be seen with the naked eye. "What we use is a scanning electron microscope and the magnification is very high. We can go up to 30, 000 in terms of magnification," she says. "So far we’re playing around with ‘can you do this from corn-based polymers?’ ‘Can you create this apartment complex with open cavities?’" Nawaby says. Nawaby has been testing CO2 and nitrogen with the polymers. The first step is to dissolve the gas. Secondly, wait until the gas is completely dispersed everywhere and it has reached equilibrium, a condition in which everything is balanced. This could take 24 or 48 hours depending on the gas. The last step is to quickly drop the pressure or raise the temperature to disrupt the equilibrium, Nawaby says. "Those little cavities get created because of this sudden thermodynamic change." Think of opening a soda bottle and watching all the bubbles race to the surface. It is these bubbles that would lock in place and form the cavities or holes that would become the home for cells. Ideally all the cavities in the scaffold should be the same size. "It crystallizes or the chains start to order themselves and it sometimes prevents you from having a very uniform apartment complex…you may have one very big…apartment and then a small one," Nawaby says. Getting that uniform structure is a process control issue. "We know that with CO2 it works. There’s no doubt it does work, but whether you’re going to get that ideal structure is going to take the work…" Nawaby says. Such work is important to Carlsson, who could potentially use a polymer structure to repair knee tissue. New knees There are two possible ways to regenerate damaged knee cartilage. The first is to put this cell-friendly foam scaffold into someone’s body. If it were put into the knee, the movement of the joint going back and forth would pump cells into the scaffold, Carlsson says. The cells could then multiply and regenerate new tissue.
Another option would be to multiply or expand the cells outside the body. Cells would be extracted from the patient and placed in a Petri dish. A cell biologist could get them to multiply. Once a good number of healthy cells were grown they could be seeded into the foam scaffold. This would become the implant that is surgically placed in the knee, Carlsson says. For these methods to work the foam scaffold has to be a perfect fit. "…If you play the game correctly you can design the size of the…holes for it so they’re big enough to let the cells wander in, but not so big that they just come straight back out again," Carlsson says. A corn-based polymer called polylactic acid is being researched to try to create a uniform scaffold. It’s a polymer that can be designed to slowly biodegrade, Carlsson says. "Biodegrade means it’s going to break down under the actions of the enzymes in your body…at the position where you implant it, so even though you’ve put cells in and it’s foam to start with, the foam is going to progressively disappear," Carlsson says. "And you can design the system so it will disappear at the same rate as the cells are generating their own matrix, their own structure." "Don’t rush out and say 'gee my knee is hurting I want this fixed right now,'" Carlsson says. "We’re talking future here."
|
|
|