|
|
Zelma Kiss is a neurosurgeon and scientist at the University of Calgary. She specializes in motor disorders, and over the past decade she has helped 68 patients regain a degree of normalcy through a procedure called deep brain stimulation.
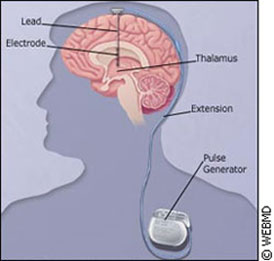
Through this process, an electrode is surgically inserted into the brain of Parkinson’s patients, and hooked up to a pacemaker in the chest. The electrode emits electric pulses that provide remarkable results. Kiss tells of a former patient, who was just 41-years-old at the time her stimulator was implanted. Like most candidates for this surgery, she was in the mid-to-advanced stage of Parkinson’s – a degenerative, hereditary disease that attacks the central nervous system. When not on her medication, she involuntarily curled up into a ball, like a scared child. “Before surgery, she would spend about half of her day in this state,” says Kiss. “The medications weren’t working for her, she couldn’t move, and she was essentially curled up on a couch.” The woman wasn’t able to rise from a chair without help, and was unable to walk on her own. When taking her prescribed medication, Levodopa (also known as L-Dopa), she exhibited abnormal movements, rocking back and forth to a peculiar rhythm. Dyskinesia, or uncontrollable movements, is a typical side effect of L-dopa, and the most visible sign of Parkinson’s. Immediate effects After the implantation surgery – though still off her medication – Kiss saw an immediate improvement in her patient. “Within a minute of it being activated, her muscles relaxed, and she began to come back to her normal self,” explained Kiss. Finally, when the two treatments were combined, something special happened. “She essentially looks normal,” says Kiss. “The stimulation works together with medication in Parkinson’s patients to essentially normalize their lives, so that they are functional throughout the waking day, as opposed to being dysfunctional for half of it.”
According to Parkinson Society Canada, approximately 100,000 Canadians are affected by this disease. Most of the time, its victims are in their sixties, but in some cases, symptoms can appear much earlier. The disease takes hold when cells in the substantia nigra region of the brain stop making dopamine. A fellow neurotransmitter to serotonin and norepinephrine, dopamine is responsible for regulating parts of the central nervous system. It sends messages to the appropriate areas of the brain, which then control movement of the body. Without dopamine, patients experience Parkinsonism; symptoms like muscle rigidity, tremors, and the slowing – or even loss – of normal movement. These symptoms get worse over time. Most often – especially in its early stages – Parkinson’s is treated with medication that replaces this missing dopamine. Unfortunately, replacing dopamine in the brain is no easy task, due to its molecular weight. It is too heavy to pass through the brain-blood barrier, so dopamine cannot access the central nervous system externally. Instead, L-Dopa is used. In use since 1968, L-Dopa is molecularly lighter, is able to pass through the brain-blood barrier, and is metabolized to dopamine. However, problems occur when the dopamine floods the brain, and enters areas where it’s not needed. This leads to dyskenesia. Deep brain stimulation Deep brain stimulation, on the other hand, is a much newer therapy. Electrical stimulation itself has been around since the 1970s, its origins in the treatment of pain.
In the late 1980s, Alim-Louis Benabid – a French neurosurgeon working out of Grenoble, France – thought of applying the technology of high-frequency electrical stimulation over an extended period of time. “We used to burn that part of the brain to suppress tremor. Instead of burning that area, he put this chronic stimulating electrode, which was previously used for pain, into this area of the brain,” explains Kiss, who studied under Benabid. Soon after, industry became interested and scientists began to have a better understanding of where the electrodes were implanted, around the sub-thalamic nucleus areas of the brain. To date, says Kiss, around 30,000 of these systems have been installed. “Before 1993, we had no good surgical options [for Parkinson’s]. This technology completely changed the way these patients are now managed,” says Kiss. Even so, there is still an air of mystery wafting around the procedure. “We still don’t really understand how electric stimulation modifies brain activity,” says Kiss. Dr. Robert Chen, a neurologist in the Department of Medicine at the University of Toronto, agrees. He says that initially the literature specu
lated the effects of stimulation were similar to that of lesion surgery. They are now beginning to realize the process is much more complicated. “We have done some studies that indicate deep brain stimulation actually activates the target area, rather than inhibiting the target area,” says Chen. “But exactly how it works, is still not exactly known. Mapping it out The procedure isn’t rocket science, but it does take a neurosurgeon or two. The initial planning phase is one of the most important parts of the surgery. Before operating, a team of neurosurgeons, neurologists, and their aids makes an external frame – an MRI – and identify standard markers on the imaging, like landmarks on a map. They then translate this into a “brain atlas” which they use as their guide for inserting the micro-electrode deep down into the brain. Next they map what types of cells they are stimulating, and the effects the stimulation produces. The patients are generally awake throughout this procedure. Kiss describes the next step.
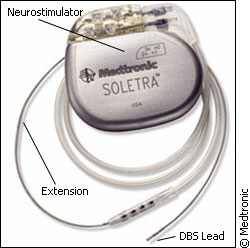
“Then we implant the device. Using simple x-rays, we take some images to confirm the electrodes are in the right place… and then we connect it to a pacemaker system, an entirely implantable system which includes the battery, includes the stimulation, and can be used then for a period of 3-5 years, until you have to replace the battery.” The planning and mapping process is key, and precision is vital. “The location is incredibly important. If you are 2-4 millimetres off, it won’t work,” says Kiss. One size doesn't fit all The surgery isn’t for all Parkinson’s cases. Both Kiss and Chen say candidates must go through a rigorous selection process, to make sure the therapy is right for them. Chen says ideal candidates are younger, with no memory problems - the surgery can make thsese problems worse. Their Parkinson's symptoms must have responded well to L-Dopa, but as a result, they have experienced unpleasant side effects. Moreover, the implants aren’t problem-free. Kiss says there are three areas where complications can arise. The first is during surgery. A small mistake can lead to bleeding, resulting in stroke or death. She says the process of implanting the electrodes is on par risk-wise with other brain surgeries, at about two or three per cent.
The second is technology-related – complications that arise out of the pacemaker itself. Infections could occur at the area of installation, or there could be wiring or battery problems. The final problem area is the most common, but least dangerous. Kiss says about 20 per cent of patients experience a decline in “executive functions.” These are tasks one would do on a normal, everyday basis, like decision-making. Chen says patients also have reported mood changes. Even without the complications, deep brain stimulation is by no means a “cure” for Parkinson’s. Relief of symptoms is often transient and varied. When the stimulator is turned off, its effects disappear. Some symptoms, like tremor, are more easily relieved than others. As well, because of Parkinson's progressive nature, the effectiveness of the implant decreases over time. And because it is a new technology the longest trial for the implants to date is only 15 years. Kiss says if the technology continues to develop as rapidly as it has in the past, we will have an exciting decade on our hands. Deep brain stimulation is also being tested in treatments for other neurological disorders, like epilepsy and depression. But right now it is advances in the technology, like rechargeable batteries and upgrades to the stimulator, which will be the next step to make this therapy really smart.
|
|
|