|
Seems quick and easy, right? But, would you try it? The glorious promise of counteracting global warming blurs any suspicion that “sprinkling” iron could be a euphemism for “dumping,” and that the technique may not only fertilize the oceans, but also contaminate them. When private companies, such as California-based Climos, announced their intent to sell carbon offsets from OIF the news raised eyebrows within the scientific community.
Charlie Trick, from the University of Western Ontario, is among the scientists who are cynical about OIF as a carbon sequestration technique. A microbiologist, Trick participated in three of the 12 major (yet small-scale) OIF experiments that have been carried out in open ocean waters since 1993. According to Trick, it’s very inexpensive to fertilize the ocean with iron. “Even though all these experiments cost more than a million dollars to run,” he says, “it only costs about $500 of iron.” Trick says OIF experiments have allowed scientists to learn more about ocean chemistry, but have not been effective at measuring the efficacy of OIF as a strategy for carbon capture and storage.
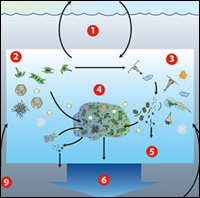
Phytoplankton, the microscopic plants that exist in the ocean but burst in quantity when iron is added, absorb carbon dioxide from the atmosphere to grow. In turn, zooplankton eat phytoplankton to grow and exhale CO2 back into the atmosphere. Eventually, the remains of decaying phytoplankton and fecal pellets from zooplankton—which contain carbon—sink into the depths. Here's the problem: Only a small amount of carbon absorbed by plankton blooms sinks deep enough into the ocean to prevent it from being released back into the air. Oceanus, a publication by the Woods Hole Oceanographic Institution, states in their special issue on OIF that only one to 15 per cent of the original carbon in surface waters sinks below 500 metres, the estimated minimum depth for effective sequestration. Based on experimental results at 200 metres depth, scientists have been able to estimate that for every ton of iron added to an ocean, 200 tons of carbon are sequestered. It is very challenging to make precise measurements, due to the nature of these oceanic experiments. The usual method involves sprinkling acidified iron sulphate into the ocean as a thin slurry. This minimizes the amount that instantly sinks below the sunlit surface waters where photosynthesis occurs. To add the iron, a ship must zigzag for 12 hours across a theoretical patch of water whose borders budge constantly in the ocean currents. In the subsequent weeks of monitoring, a ship spends an average of 12 hours per day mapping out the boundaries of the plankton bloom. Blooms become practically undetectable after a few days because the added iron slurry quickly dilutes, sinks, and reacts with seawater. Moreover, local currents have an effect on the bloom size. According
to Uncertainties surrounding how to best conduct OIF and its effectiveness as a carbon sequestration strategy are significant. Not to mention the uncertainties surrounding the unintended consequences of fertilizing the oceans with iron. How much iron is too much? The 12 small-scale experiments have not detected any negative effects to date, but there’s no guarantee larger experiments will either, because any effects are likely to dissipate in the oceans’ immensity. In spite of opposition within the scientific community, private companies want to further advance research on OIF through larger-scale experiments while cashing in some bucks from carbon offsets at the same time. “We are convinced that, as yet, there is no scientific basis for issuing such carbon credits for OIF,” wrote a group of 16 scientists in a January article in the journal Science.
In fact, OIF doesn’t currently meet the standards for carbon-credit trading in markets regulated by the Kyoto Protocol. However, there are voluntary markets, such as the Chicago Climate Exchange, where projects don’t have to abide by the strict regulations imposed by Kyoto in order to sell carbon offsets to concerned individuals and companies who have no obligation to reduce emissions. Many scientists believe further experiments should be carried out. But many, including biological oceanographer John Cullen from Dalhousie University, also believe it is necessary to proceed with caution. “Who is watching after monitoring how the whole ocean is changing?” says Cullen, adding that studying oceanic changes is extremely challenging. “It’s a responsibility, if you want to go forward with [ocean iron fertilization], to show that the effects can be predicted and that those predictions can be verified with real measurements.”
|
|
|