In November, Preseau became one of approximately 500,000
women who are pregnant in Canada each year and must wade
through heaps of ambiguous research, wondering "Is
this safe for my baby?"
 |
| Conflicting drug research means difficult
decisions for pregnant women. |
"I never used to think about how everything I did
was affecting me,"says
Preseau, "but now it's all about the baby."
Excited to see a tiny set of 10 fingers and toes in August,
Preseau is trying to avoid exposing her first baby to harmful
substances that could cause birth defects.
Despite her doctor prescribing Diclectin, an anti-nausea
drug used during pregnancy for 40 years in Canada, Preseau
is resistant to join the estimated 80 per
cent of pregnant women who use medication.
| 'A pregnant woman might
say 'I don't want to take anything' but she could be
putting herself and her baby at even more risk' |
"I'm not against it, but if I don't
desperately need it, it's better not to take anything," says
Preseau, who carries blue plastic bags in her wallet for
emergency nausea bouts. "I'll try
everything else before I take medication."
According to Motherisk, a Toronto-based research organization
that counsels pregnant women on drug safety, women and their
doctors must find a balance that will keep the mother healthy
and the developing foetus at the lowest risk.
Motherisk fields about 200 calls each weekday about the
safety of drugs from over-the-counter cold remedies, to prescription
medications for mental illness.
Confusion prevails
With more research accessible than ever why are pregnant
women still hesitant?
"It is very challenging," says Ellen Reynolds,
director of communications for the Canadian Women's
Health Network. "There is a lot of conflicting
information out there."
Barbara Mintzes, an assistant professor in the department
of anaesthesiology, pharmacology and therapeutics at the
University of British Columbia says some scientific studies
are little more than marketing aids and add to the confusion.
"You'll have
huge differences in what different studies might say partly
because it's
an area where there are strong financial interests at play,"
says Mintzes. "For trials sponsored by drug manufacturers,
some that are planned in a way that it's clear the
manufacturer will show an advantage for their drug."
Reynolds also
says memories of the "thalidomide tragedy" make
pregnant women think twice before popping pills.
It's one reason Preseau says she doesn't want
to take Diclectin despite her doctor's recommendation
and supporting research. "I just can't get
out of my mind the thalidomide babies," she says. "They
said 'it's okay' and it turned out it
really wasn't okay."
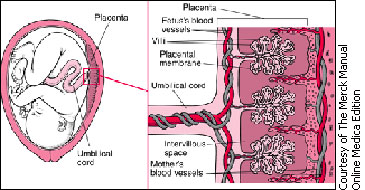 |
Drugs in the mother's blood can
cross the placental membrane into blood vessels in
the villi and pass through the umbilical cord to the
fetus. |
Reynolds says pregnant women should err on the side of
caution. "With
thalidomide the effects were immediate at birth but with
DES, it was subtle so the effects were only revealed 15 to
20 years later. We can't be sure what we think
is safe actually is," she says, "Precaution
is the only sensible way to go." Thorough, long-term
testing is needed to prevent another medical mistake, she
says.
Finding the balance
While most researchers say pregnant women should be informed
about which medications they use, not all agree that avoiding
medication is the safest bet.
"Not all drugs are thalidomide," says Myla Moretti,
assistant director of Motherisk. She says though using
drugs can be dangerous, "the
most dangerous thing a woman can do is make drastic decisions
out of fear of the unknown ... Sometimes not treating
is more dangerous than treating."
| 'I just can't
get out of my mind the thalidomide babies. They says
'it's okay' and
it turned out it really wasn't okay.' |
"Some
conditions, if left untreated, can put the pregnancy at risk,"
Moretti says. "A pregnant woman might say 'I don't
want to take anything' but she could be putting herself and
her baby at even more risk." Even
if a mom-to-be does everything right, she adds there's
still a three per cent chance to have a birth defect.
Ethical considerations
Another reason why little is known about safe drug use
during pregnancy, says Mintzes, is because researchers are
ethically and legally reluctant to test drugs on pregnant
women. "For
pharmaceutical companies the liabilities are higher for
pregnant women," she says.
 |
| Thalidomide is now used in treating
leprosy. |
According
to Moretti, by the time they hit the market, most drugs
have not been tested for safety (or even effectiveness,
adds Mintzes) in pregnancy. This gives women who become
pregnant access to thousands of medications, few of which
have actually been proven safe or effective during pregnancy. Moretti
says medicating is a judgement call that must be made on
a case-by-case basis between a woman and her doctor by balancing
the potential risks with the potential benefits.
Preseau says she is happy to keep her prescription in her
wallet for now right next
to the blue plastic bags.
"You can't believe everything you read, especially
with something so important," says Preseau. "You
just have to take little bits and pieces of information from
everywhere and hope you are doing the right thing."
Front Page Courtesy of The Anti-Stress Blog for Women
|

